
The concentration of the gas in a liquid is also dependent on the solubility of the gas in the liquid. The greater the partial pressure of the gas, the greater the number of gas molecules that will dissolve in the liquid. Henry’s law states that the concentration of gas in a liquid is directly proportional to the solubility and partial pressure of that gas. Henry’s law describes the behavior of gases when they come into contact with a liquid, such as blood. In addition, the greater the partial pressure difference between the two areas, the more rapid is the movement of gases. A gas will move from an area where its partial pressure is higher to an area where its partial pressure is lower. Recall that gases tend to equalize their pressure in two regions that are connected. Partial pressure is extremely important in predicting the movement of gases.
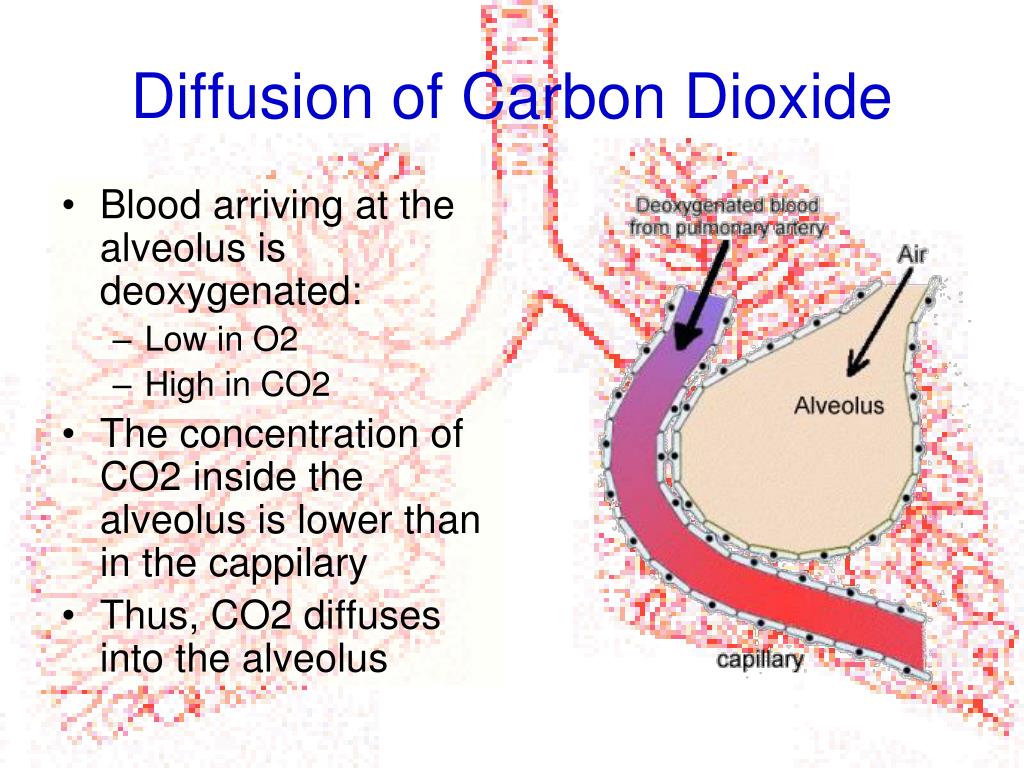
The sum of the partial pressures of all the gases in a mixture equals the total pressure. Dalton’s law describes the behavior of nonreactive gases in a gaseous mixture and states that a specific gas type in a mixture exerts its own pressure thus, the total pressure exerted by a mixture of gases is the sum of the partial pressures of the gases in the mixture.įigure 22.21 Partial and Total Pressures of a Gas Partial pressure is the force exerted by a gas. Total pressure is the sum of all the partial pressures of a gaseous mixture. For example, in the atmosphere, oxygen exerts a partial pressure, and nitrogen exerts another partial pressure, independent of the partial pressure of oxygen ( Figure 22.21). Partial pressure ( P x) is the pressure of a single type of gas in a mixture of gases. For example, the atmosphere consists of oxygen, nitrogen, carbon dioxide, and other gaseous molecules, and this gaseous mixture exerts a certain pressure referred to as atmospheric pressure ( Table 22.2). In natural systems, gases are normally present as a mixture of different types of molecules. Gas molecules exert force on the surfaces with which they are in contact this force is called pressure. In addition to Boyle’s law, several other gas laws help to describe the behavior of gases. In order to understand the mechanisms of gas exchange in the lung, it is important to understand the underlying principles of gases and their behavior. It is through this mechanism that blood is oxygenated and carbon dioxide, the waste product of cellular respiration, is removed from the body. At the respiratory membrane, where the alveolar and capillary walls meet, gases move across the membranes, with oxygen entering the bloodstream and carbon dioxide exiting. Pulmonary ventilation provides air to the alveoli for this gas exchange process. The purpose of the respiratory system is to perform gas exchange.

Describe the process of internal respiration.
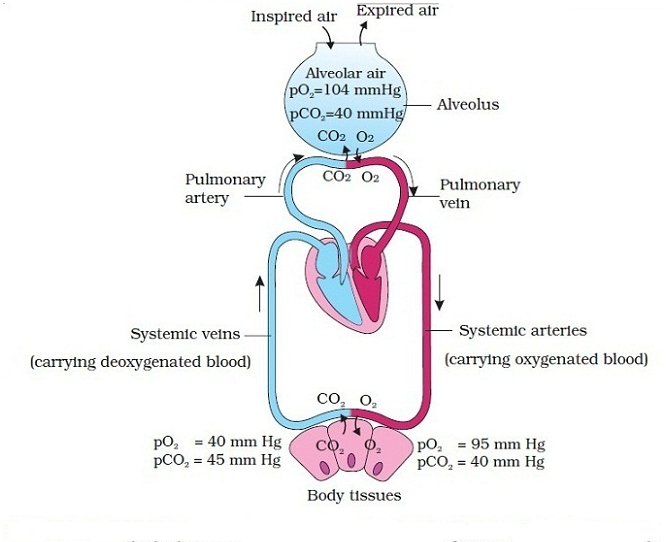
Discuss the process of external respiration.Discuss the importance of sufficient ventilation and perfusion, and how the body adapts when they are insufficient.Describe the mechanisms that drive gas exchange.Compare the composition of atmospheric air and alveolar air.By the end of this section, you will be able to:


 0 kommentar(er)
0 kommentar(er)
